Urea Cycle Disorder Treatment Market Outlook 2035: Rising to US$ 771 Mn at 3.5% CAGR | Transparency Market Research
Urea Cycle Disorder Treatment Market to reach US$ 771.0 Mn by 2035, expanding at 3.5% CAGR, driven by rising prevalence, product approvals, and novel therapies.
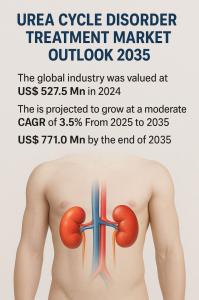
WILMINGTON, DE, UNITED STATES, September 5, 2025 /EINPresswire.com/ -- The global urea cycle disorder (UCD) treatment market is entering a new phase of steady expansion, driven by advancements in diagnosis, innovative therapeutic approaches, and increased awareness of this rare but life-threatening condition. Valued at US$ 527.5 million in 2024, the market is projected to grow at a moderate CAGR of 3.5% from 2025 to 2035, surpassing US$ 771.0 million by 2035. While UCD remains a relatively rare genetic condition, its growing prevalence, combined with supportive regulatory frameworks and medical breakthroughs, is expected to reshape the treatment landscape.Access key findings and insights from our Report in this sample - https://www.transparencymarketresearch.com/sample/sample.php?flag=S&rep_id=86533
Market Size and Growth
The UCD treatment market, though niche, represents a vital segment of the rare disease therapeutic landscape. With an estimated incidence of 1 in 35,000 births in the U.S. alone, the disorder places a significant burden on patients, caregivers, and healthcare systems. As of 2024, market size stood at US$ 527.5 million, primarily supported by demand for nitrogen-scavenging medications, specialized medical foods, and liver transplantation procedures.
Looking ahead, the market’s trajectory reflects both opportunity and challenge. On one hand, the projected growth to US$ 771.0 million by 2035 underscores the increasing recognition of UCD and the steady inflow of new treatment options. On the other hand, the modest CAGR of 3.5% highlights the rarity of the condition, limiting the patient pool compared to other metabolic or genetic disorders. Nevertheless, with investments in cutting-edge therapies such as gene therapy and enzyme replacement, the growth outlook remains positive.
Market Segmentation
Segmentation of the UCD treatment market provides insight into areas of highest demand and innovation. By disease type, Ornithine Transcarbamylase (OTC) deficiency dominates due to its higher prevalence and severity compared to other enzyme deficiencies. OTC deficiency leads to rapid ammonia accumulation, often requiring immediate intervention, and therefore fuels significant demand for both acute and long-term treatment solutions.
By treatment type, the market is segmented into drugs (sodium benzoate, sodium phenylbutyrate, glycerol phenylbutyrate, and emerging nitrogen scavengers), dietary management, dialysis, liver transplantation, and novel therapies such as gene therapy. Among these, nitrogen-scavenging drugs continue to hold a major share, but liver transplantation remains the definitive treatment in severe cases. Gene therapy, though still in development, is expected to revolutionize long-term outcomes by correcting the underlying enzymatic defect.
From a distribution channel perspective, hospital pharmacies dominate due to the need for specialized care and monitoring. Given the acute nature of metabolic crises in UCD, treatments are often administered in controlled clinical environments. Retail pharmacies and online channels play a smaller but growing role, particularly in providing long-term medications for stable patients.
Regional Analysis
Geographically, North America leads the global UCD treatment market, supported by advanced healthcare infrastructure, higher diagnostic rates, and favorable regulatory policies. The United States, in particular, benefits from orphan drug incentives, comprehensive insurance coverage, and the presence of major biopharmaceutical players, making it the largest revenue-generating market for UCD treatments.
Europe follows closely, with countries like Germany, France, and the U.K. investing heavily in rare disease treatment frameworks. The European Medicines Agency (EMA) has also provided fast-track approvals for therapies addressing UCD, driving access across the continent.
Meanwhile, Asia-Pacific represents an untapped growth frontier. While diagnosis and awareness levels remain relatively low, improvements in genetic testing and expanding healthcare access in countries like China, Japan, and India are expected to boost demand. Collaborations between multinational drug makers and local healthcare systems are accelerating availability, making the region a promising contributor to future market expansion.
Market Drivers and Challenges
Key Drivers
1. Rising Prevalence and Diagnosis – Advances in genetic testing and neonatal screening are leading to earlier and more frequent diagnoses, directly boosting the demand for UCD treatments.
2. Product Approvals and Innovation – Regulatory agencies are accelerating approval timelines for life-saving therapies, ensuring faster market entry and broader treatment options.
3. Increased Awareness – Public health campaigns and medical education programs are raising awareness, improving early intervention, and expanding the patient pool eligible for treatment.
Challenges
Despite strong growth drivers, the market faces critical hurdles. The high cost of treatments, particularly liver transplantation and emerging gene therapies, limits accessibility, especially in low- and middle-income regions. Limited patient populations also restrict commercial incentives for pharmaceutical companies, while the complexity of long-term disease management poses challenges for both caregivers and healthcare providers. Addressing these issues requires not only medical innovation but also supportive reimbursement policies and global collaboration.
Competitive Landscape
The UCD treatment market is moderately consolidated, with several global and regional players actively pursuing innovation. Key companies include Bausch Health Companies Inc., Eurocept Pharmaceutical Holding, Zevra Therapeutics, Ultragenyx Pharmaceutical, Aeglea BioTherapeutics, Arcturus Therapeutics, Orpharma Pty Ltd., Abbott, Nestle SA, Mead Johnson & Company, Boehringer Ingelheim International, and CAMP4 Therapeutics.
These firms are focusing on R&D investment, product launches, mergers and acquisitions, and partnerships to strengthen their market positions. Ultragenyx and Arcturus, for example, are at the forefront of gene therapy development, while Abbott and Nestle remain leaders in the nutritional management space. Competitive strategies also include expanding treatment access in emerging markets and offering pricing models tailored to healthcare systems.
Buy this Premium Research Report for exclusive, in-depth insights - https://www.transparencymarketresearch.com/checkout.php?rep_id=86533<ype=S
Future Outlook
The future of the UCD treatment market lies at the intersection of biotechnology innovation and global healthcare accessibility. By 2035, the market is projected to exceed US$ 771.0 million, with gene therapies potentially transforming the standard of care. Broader neonatal screening programs are expected to increase diagnosis rates, while patient advocacy and rare disease funding initiatives will improve awareness and treatment adoption.
More Trending Reports by Transparency Market Research –
Technology Spending on Revenue Cycle Management Market - https://www.transparencymarketresearch.com/technology-spending-revenue-cycle-management.html
Thermal Cycler Market - https://www.transparencymarketresearch.com/thermal-cycler-market.html
Battery Cyclers Market - https://www.transparencymarketresearch.com/battery-cyclers-market.html
Revenue Cycle Management Market - https://www.transparencymarketresearch.com/revenue-cycle-management-market-report.html
Anxiety Disorders and Depression Treatment Market - https://www.transparencymarketresearch.com/anxiety-disorders-depression-treatments.html
Treatment for Syndromes of Progressive Ataxia and Weakness Disorders Market - https://www.transparencymarketresearch.com/syndromes-progressive-ataxia-weakness-disorders.html
Autism Spectrum Disorder Treatment Market - https://www.transparencymarketresearch.com/autism-spectrum-disorder-treatment-market.html
Movement Disorder Market - https://www.transparencymarketresearch.com/movement-disorder-market.html
Thyroid Gland Disorders Treatment Market - https://www.transparencymarketresearch.com/thyroid-gland-disorder-market.html
Application Lifecycle Management (ALM) Market - https://www.transparencymarketresearch.com/application-lifecycle-management-market.html
Recycled Metal Market - https://www.transparencymarketresearch.com/recycled-metal-market.html
Tricycles Market - https://www.transparencymarketresearch.com/tricycles-market.html
Recycled Aluminum Market - https://www.transparencymarketresearch.com/recycled-aluminum-market.html
About Transparency Market Research
Transparency Market Research, a global market research company registered at Wilmington, Delaware, United States, provides custom research and consulting services. Our exclusive blend of quantitative forecasting and trends analysis provides forward-looking insights for thousands of decision makers. Our experienced team of Analysts, Researchers, and Consultants use proprietary data sources and various tools & techniques to gather and analyses information.
Our data repository is continuously updated and revised by a team of research experts, so that it always reflects the latest trends and information. With a broad research and analysis capability, Transparency Market Research employs rigorous primary and secondary research techniques in developing distinctive data sets and research material for business reports.
Contact:
Transparency Market Research Inc.
CORPORATE HEADQUARTER DOWNTOWN,
1000 N. West Street,
Suite 1200, Wilmington, Delaware 19801 USA
Tel: +1-518-618-1030
USA – Canada Toll Free: 866-552-3453
Website: https://www.transparencymarketresearch.com
Email: sales@transparencymarketresearch.com
Atil Chaudhari
Transparency Market Research Inc.
+1 518-618-1030
email us here
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.